This paper investigates the effectiveness of three mineral admixtures in reducing the expansion of concrete due to alkali-silica reaction. Three types of aggregates, two natural sands and one crushed sand, are used for preparing mortar bars. These aggregates have been petrographically examined according to ASTM C295 standard and have been found out harmful in respect to ASTM C 1260. In this study, admixtures include one class F fly ash, a condensed silica fume, blast furnace slag. The performance of these three admixtures were compared by examining the expansion due to alkali-silica reaction (ASR). The aggregate and cement mixtures were tested with accelerated mortar bar test procedures included %10 and %20 replacement by mass of cement. Condensed silica fume tested with the same test procedures but only %10 replacement by mass of cement because its optimum use of silica fume in concrete. The results of 14-day experimental measures showed that the average maximum length of extended control mortar bars was observed to be % 0.56, while the lowest average increase was %0.20 of the length. In the mortar bar silica fume replacement was %10 by mass of cement and length change was seen to be %0.04 in the mortar bar. According to findings of this study, %10 substitution level of silica fume and %20 substitution level of fly ash and blast furnace slag was highly effective in inhibiting the alkali-silica reaction. The expansion of test specimens, in which mineral admixtures are used, were within acceptable limits.
Keywords: Alkali-silica reaction (ASR), Silica fume (SF), Fly Ash (FA), Blast Furnace Slag (BFC).
1 Introduction
Alkali silica reaction is the reaction between alkali hydroxide in Portland cement and reactive silica present in the aggregates. This reaction often results in significant concrete expansion and cracking which ends with the failure of the concrete structure (Stanton,1940). ASR reaction needs several components to occur; alkali, reactive aggregate and water. Especially if there isn’t any water ASR jels doesn’t expand and no reaction will occur (Hobbs,1980). Water and moisture is highly essential for ASR expansion.
ASR is a serious concrete detorioration and a serious problem throught the World. There are many theories proposed by different researchers that explains the mechanism of the reaction. One theory explains the ASR as following; 1.OH- attacks the aggregate and interrupts its dissolution, 2. dissolved silica reacts with alkalis (Na+ or K+), 3a. expansion of concrete is due to osmotic pressure generated by alkali silica jels in cement paste (Hansen,1944), 3b the expansion is a consequence of the formation of cracks and widening of cracks due to mechanical pressure exerted by the reaction products (McGowan et. all, 1952; Vivian 1951), the expansion of concrete depends on if the reaction formed swelling alkali silica gel or non swelling type. Other researchers explain the reaction by other theories and it seems none or these theories generally accepted.
Despite the complexity of the alkali silica reaction, there are four options to prevent or control harmful ASR expansion on concrete structures; 1. avoid reactive aggregate, 2. use low alkali cement, 3. use a chemical additive 4. partially replace high alkali cement by supplemantary material. Mitigation techniques with ASR were investigated around the world by researchers. The last option has attracted much attention by researchers because abundant supplemantary materials are available for use in concrete and mortar (Hobbs, 1989; Hogan, 1985). Today it is well known that supplemantary materials called mineral admixtures such as fly ash, silica fume and blast furnace slag have been used succesfully to control the ASR expansion (Xu et. all.,1995; Rangaraju et. all.,2009; Andiç, 2002). Various proposed mechanisms have been tried to explain mineral admixtures role in ASR expansion. These mechanisms have been studied by many researchers, which include alkali dilution, reducing permeability and alkali adsoprtion by extra CSH gels formed with pozzolonic reaction (Berube et. all., 1992; Thomas et. all.,1996; Durand 1991). However, all of these hypothesis are still in dispute because of different views on the ASR mechanisms.
Each of these mineral admixtures shows significant performance reducing ASR. Class F fly ash reduces concrete permeability (Ellis,1992) and in replacement amounts around %25 have been shown to significantly mitigate ASR even in marine environments for concretes whose w/c ratio are below 0.5 (Malhotra et. all., 1994; Touma et. all., 2000; Berube et. all., 2000). Silica fume has also been proved to mitigate ASR, for example in %10 cement replacement level silica fume reduce expansions to a level close to %20 fly ash cement replacement (Touma et. all.,2000). Blast furnace slag was found effective in reducing ASR expansion for example mortar bars containing slag showed lower expansions at 14 days than their control specimens. (Rangaraju et. all., 2009.)
2 Materials
One type of cement and three types of mineral admixtures with three types of aggregates are used in preparing mortar bars in this study. Totally 12 mixes and 24 mortar bars in total are prepared to use in the tests.
2.1 Cement and Mineral Admixtures
A high alkali cement with a Na2O equivalent of %0.83 is used in the study (ASTM Type I) and shown in equation 1. The chemical composition of the cement is provided in Table 1. One type of F class fly ash, blast furnace slag and silica fume is used and chemical composition of these three type of mineral admixtures are provided in Table 1. Mineralogical composition of cement is provided in Table 2.
2.2 Aggregates
Three types of aggregates are used in this study. Their origin and mineral composition are different from each other. Mineral composition of aggregates are taken from petrographic examination results which were conducted in accordance with ASTM C227.
2.2.1 Highly Reactive Natural Sand
This aggregate is a natural sand obtained from Sakarya river. Hereafter this aggregate is named as S1 aggregate. Tests performed according to ASTM C1260 standard, 14 days expansions are recorded eventually average of the expansions are calculated as %0,60. Aggregates that shows higher expansion than %0.2 at the end of 14 days is defined high reactive aggregate by researchers (Touma et. all, 2000). Considering the average of 14 days of expansion S1 aggregate can be included in this highly reactive aggregate class. Also mineralogical composition of S1 aggregate is shown in Table 4.
2.2.2 Highly Reactive Crushed (Sand)
Aggregate Stone
This aggregate is a crushed aggregate stone obtained from İstanbul region. Hereafter this aggregate is named as S2 aggregate. Tests performed according to ASTM C1260 standard, 14 days expansions are recorded eventually average of the expansions are calculated as %0,32. Aggregates that shows higher expansion than %0.2 at the end of 14 days is defined high reactive aggregate by researchers (Touma et. all, 2000). Considering the average of 14 days of expansion S2 aggregate can be included in this highly reactive aggregate class. Also mineralogical comp
2.2.3 Highly Reactive Natural Sand
This aggregate is a natural sand obtained from İstanbul region. Hereafter this aggregate is named as S3 aggregate. Tests performed acoording to ASTM C1260 standard, 14 days expansions are recorded eventually average of the expansions are calculated as %0,24. Aggregates that shows higher expansion than %0.2 at the end of 14 days is defined high reactive aggregate by researchers (Touma et. all, 2000). Considering the average of 14 days of expansion S3 aggregate can be included in this highly reactive aggregate class. Also mineralogical composition of S1 aggregate is shown in Table 6.
3 Experimental Set-Up
An experimental matrix includes 18 mixes in total 1) 3 control mixes, 2) 3 with %10 replacement level of fly ash, 3) 3 with %20 replacement level of Fly ash, 4) 3 with %10 replacement level of blast furnace slag, 5) 3 with %20 replacement level of blast furnace slag, 6) 3 with %10 replacement level of silica fume. Replicates are two for each mix therefor we have two mortar bars for each mix.
3.1 Sample Preparation
The composition of the control mortar bar was 1 part cement to 2.75 parts of aggregate and the water content ratio by mass 0,47 for all samples in accordance with ASTM C1260 standard. Mortar bars (25 mm x 25 mm x 285 mm) were prepared to test ASR expansion.
3.2 Test Methods
Comparative evaluation of test methods for assessing the effectiveness of mineral in suppressing expansion due to alkali-aggregate reaction have been reported by other authors. (Davies et. all., 1987; Berube et. all., 1992). It is suggested accelerated mortar bar may give a good indication of the effectiveness of mineral admixtures reducing expansion in concrete due to ASR. ASTM C1260 (Accelareted Mortar Bar Method) test procedure is conducted in this study. In this test, mortar bars (25 mm x 25 mm x 285 mm) containing aggregate are exposed to pure water at 90 oC for one day as an initial curing and comparator reading is taken as an initial length. After that mortar bars were exposed to 1N sodium hydroxide solution at 80ºC temperature for 14 days. Change in length of mortars were monitored periodically on 1. Day, 3. Day, 7. Day and 14. Day. In this investigation, length change measurements on mortar bars weren’t taken longer than 14 days because aggregates showed reactivity and no longer measurements needn’t to be taken. According to the standard mortar bar expansion greater than %0,2 at 14 days indicates a potentially reactive aggregate. In situations where mortar bars expansion ranges between 0,1 and 0,2, additional confirmation is necessary to determine the reactivity of the aggregates by other test methods.
4 Results and Discussion
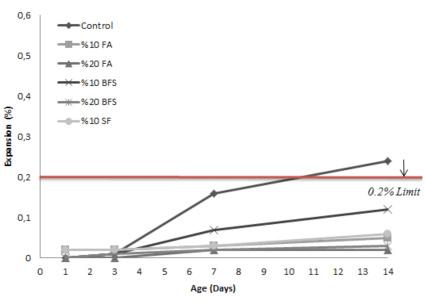
The influence of mineral admixtures on ASR expansion is shown in figure 2,3,4. In general it was found that the degree of expansion decreased as the level mineral admixtures increased. Based on the results of accelerated mortar bar tests, in samples prepared using S1 aggregates it appears that %10 replacement level of cement by fly ash and slag samples have the expansions lower than control samples but were not in acceptable limits. For all aggregates it appears that %20 replacement level of fly ash and slag successfully lower down the expansions under acceptable limits. %10 replacement level of silica fume was highly effective mitigating ASR. Samples with silica fume showed lower expansions under acceptable limits. It can be concluded that using slag and fly ash at %20 cement replacement level and silica fume at %10 replacement level seems effective in mitigating expansions where as using slag and fly ash at %10 cement replacement level wasn’t enough to suppress them.
5 Conclusions
The main purpose of this study, was to compare the relative effectiveness of different mineral admixtures in mitigating ASR expansion. All the samples with admixtures show less expansion than that of control mortar bar. Mortar bars containing fly ash and slag at levels of replacement up to %10 failed to control ASR expansion of S1 aggregate. Fly ash and slag were effective at %20 replacement level and silica fume was very effective at %10 replacement level. Mortar bars containing fly ash, slag and silica fume at %10 and %20 levels of replacement appears to be effective to control ASR expansion of S2 and S3 aggregate. This findings are coincide with the evidence from length change measurements. Of all the mixtures tested, silica fume is the most effective material mitigating ASR expansion although the SiO2 content is much higher that other mineral admixtures.
References
Andiç, Ö., (2002). Controlling alkali silica reaction by using mineral and chemical additives. Ege University, Institute of Natural Sciences, Msc Thesis.
Berube, M.A. and Dunchesne, J. (1992). Does silica fume merely postpone expansion due to Alkali-Aggregate Reactivity. 9th International Conference on Alkali-Aggregate Reactions in Concrete, London, UK.
Berube, M.A., Durand, B., Vezina, D., Fournier, B. (2000). Alkali aggregate reactivity in Quebec (Canada), Canadian Journal of Civil Engineering, Vol. 27, pp.226-245.
Durand, B. (1991). Preventive measures against Alkali-Aggregate Reactions: A petrographic and Alkali-Aggregate Reactivity, Canmet , Course Manual, EMR:399, Ottowa, Canada.
Ellis, W.E., (1992). For durable concrete fly ash doesn’t replace cement, Concrete International, Vol. 14, No.7, pp. 47-51.
Davies, G., Oberholster, R.E., (1987). Use of the NBRI accelerated test to evaluate the effectiveness of mineral admixtures in preventing the alkali-silica reaction, Cement and Concrete Research, Vol. 17, No.1, pp.97-107.
Hansen, W.C. (1944). ACI Journal, Proceedings 40, pp 213.
Hobbs, D.W. (1980). Influence of mix proportions and cement alkali content upon expansion due to alkali silica reaction, Cement and Concrete Association, Technical Report, No.534.
Hobbs, D.W. (1989). Effect of mineral and chemical admixtures on alkali-aggregate reaction, 8th International Conference on Alkali-Aggregate Reaction, Proceedings, Kyoto, Japan.
Hogan, F.J. (1985). The effect of blast furnace slag cement on alkali-aggregate reactivity: a literature review, Cement and Concrete Aggregates, Vol. 7, No.2, pp.100-107.
Malhotra, V.M., Ramezanianpour, A.A. (1994), Fly Ash in Concrete, CANMET, MSL 94-45(IR), Ottowa, Canada.
McGowan, J.K., Vivian H.E. (1952), Australian Journal of Applied Sciences, Vol. 3, pp.228.
Rangaraju, P.E., Prangar E. and Desai J. (2009). Effectiveness of Fly Ash and Slag in Mitigating Alkali-Silica Reaction Induced by Deicing Chemicals, Journal of Materials in Civil Engineering, ASCE, Vol.21, No.1.
Stanton, D.E. (1940). The Expansion of Concrete Through Reaction Between Cement and Aggregate, Proc. American Society of Civil Engineers, 66:1781-1811.
Thomas, M.D.A. (1996). Summary of Bre Research on the Effect of Fly Ash on Alkali-Silica Reaction in Concrete, 10th Inter Symposium of Alkali Aggegate Reactions in Concrete, Melbourne, Australia.
Xu, George J.Z, Watt, Daniel F. and Hudec, Peter P. (1995) Effectiveness of Mineral Admixtures in Reducing ASR Expansion, Cement and Concrete Research, Vol. 25, No. 6, pp.1225-1236.
Touma, W.E., Suh, C., Gowler, D.W., Carrasquillo, R.L., and Folliard, K.J. (2000). Alkali-silica reaction in portland cement concrete: testing procedures and mitigation methods, Proc. 11th International Conference on Alkali- Aggregate Reaction, Ed. Berube, M.A., Fournier, B., Durand, B., pp. 513-522, Quebec.
Vivian H.E. (1951), Australian Journal of Applied Sciences, Vol. 2, pp.108.